IMPROVING MEDICAL DEVICE CLEANING WITH ENZYMES
How Biology Can Improve Hygiene at Hospitals?
The overall direct costs of hospital-acquired infections (HAI)s to US hospitals ranges from $28 billion to $45 billion1, while it is also estimated that HAIs claim about 72,000 lives per year2.
While there could be many ways to lower HAI, cleaning surgical instruments is a critical part of preventing them and enzymes are a key component. With the proper use of enzymes, we can enhance cleaning.
What are Enzymes?
Enzymes are proteins that act as catalysts, meaning they speed up chemical reactions. They are found everywhere — from the bottom of
the ocean to your backyard, and even inside our bodies.
Where are Enzymes Used Today?
For thousands of years, humans have used enzymes baking and making cheese3. While they are still employed for those purposes, the science of enzymes has evolved in leaps and bounds. This has created opportunities for new applications in many industries.
Now, enzymes are used to manufacture a variety of everyday products like sugar, yogurt, textiles and ethanol. Enzymes are also routinely added to detergents to help remove stains from fabrics, caked-on food from dirty dishes, and patient soils from surgical instruments, including endoscopes.
Use of Enzymes in Medical Device Cleaning
Enzymatic detergents designed specifically for cleaning reusable medical devices have been around for decades. Studies have demonstrated their improved performance over non-enzymatic detergents in this application4.
Enzymes are
• Biomolecules that speed up reactions and certain varieties specifically target the organic matter commonly found in medical-type soils.
• Gentle on delicate medical instruments & devices.
• Biodegradable.
• Able to be optimized to ensure proper cleaning by monitoring key factors such as temperature, dose, and time.
Enzymatic detergents are also referenced in multiple industry standards such as AAMI ST79 and the guidelines established by the FDA and the CDC5-6.
Optimize Medical Cleaning with Enzymes
Inadequate cleaning of medical instruments can have serious consequences, not only major costs for hospitals but also the potential loss of lives. Current medical device cleaners, meeting today’s cleanliness guidelines may not show effective performance on clinical soils.
Enzymatic detergents, designed specifically for cleaning reusable medical devices, have been used for several decades and are supported by industry studies demonstrating improved performance over non-enzymatic detergents.
• Inadequate cleaning before sterilization is the cause for 34% of the delays in operating room (OR)7 • These delays cost $100M and 43,000 hours per year in the US alone8. • Hospital acquired infections (HAI) result in $30B in direct medical and insurance costs and cause 72,000 deaths per year9.c
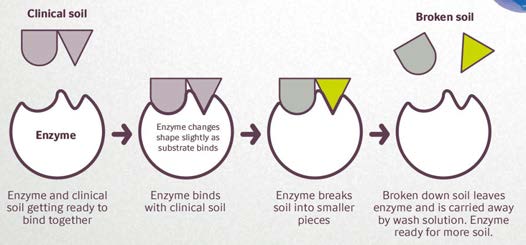
Resim 1: Enzimler klinik lekeler üzerinde nasıl çalışır?
Figure 1: How do enzymes work on clinical soils?
How Do Enzymes Work on Clinical Soils?
Enzymes work in synergy with surfactants, or wetting agents to aid in the complete removal of clinical soils from surgical instruments. Depending on the type of enzyme used, enzymatic detergents can break down soils under a wide range of temperatures.
And, they can also function at different pH levels, from neutral to alkaline conditions. Added to a detergent, enzymes enhance cleaning efficacy and are also quite relevant for multiple hospital applications, from pre-cleaning at ‘bedside’ to automatic washing in the hospital’s sterile processing department.
Research Study
Enzymatic Impact on Medical Instrument Cleaning
Novozymes conducted a study in collaboration with Stryker Corporation on the cleaning efficacy of standard enzymatic, optimized enzymatic and non-enzymatic cleaning solutions. Surgical blades containing dry clinical soils were soaked in different detergent solutions for 10 minutes and then rinsed with water.
Pictures of the blades were taken before and after cleaning. ProReveal, a highly sensitive fluorescence-based protein detection test was also performed before and after to generate fluorescent images.
What did We Find?
Under visual analysis, the optimized enzymatic detergent outperformed the non-enzymatic and standard detergents, leaving no visual residue in the blades tested. This result can be explained by the utilization of next-generation enzymes and increased dosage compared to the standard enzymatic detergent.
The subsequent fluorescent analysis confirmed this outcome. The optimized enzymatic detergent leaves no fluorescent residue after washing, while some fluorescence can still be observed in the blades washed with a standard enzymatic detergent.

Key Take-Away
Optimized enzymatic detergents provide an improvement over ’industry standard’ cleaning guidelines which can improve rewash rates, decrease waste and costs to hospitals, and improve patient outcomes.
Enzyme performance can be optimized through the using of targeted enzymes, at the proper dosage and cleaning parameters. These benefits can be directly observed with the minimization or elimination of the risk of dirty instruments.





