Summary
Two component isocyanate (2C NCO) crosslinked polyacrylate films yield a unique combination of properties, such as excellent mechanical and chemical resistances, and very good film formation. For waterborne 2C NCO curing applications, dedicated polyols and polyisocyanates have been developed.
In this work solvent free secondary emulsions were prepared containing different OH-contents. Film formation of these binders proves to be excellent, resulting in high barrier properties. Properties such as drying speed, hardness and chemical and mechanical resistances were studied.
Against water-based stains, such as coffee, red wine or 48% ethanol, 2% OH containing binders already show excellent barrier properties when crosslinked with a hydrophilic isocyanate crosslinker.
For more demanding resistances such as MEK double rubs or marker pens, higher hydroxyl contents are needed, of up to 4 or 5 wt-%.
Although the properties of 2C NCO curing acrylic resins are very good compared to 1C acrylic resins, their use in flooring applications is limited. This is mainly due to the limited maximum film thickness and the expected lack of thermoplastic behavior of the acrylic copolymer.
In this paper we show that hydroxyl functional acrylic binders can be used in flooring applications to boost the performance of waterborne urethanes coatings, when crosslinked with polyisocyanates, thus combining the best of both worlds.
Introduction
Waterborne binders for two component polyisocyanate (2C NCO) crosslinking polyacrylate coatings are a very attractive alternative to their solventborne counterparts, as they comply with legislation and do not require any labelling. However, they suffer from two distinct disadvantages.
Firstly, in solventborne coatings, polymer and crosslinker are both soluble in the continuous phase. In waterborne coatings, however, polymer and crosslinker have to be emulsified in water, and have to mix after film formation. Especially in the case of the crosslinker this can lead to serious issues .
When hydrophobic crosslinkers are selected, high shear needs to be applied for good emulsification to prevent inferior film properties. Very often, the hydrophobic crosslinker is dissolved in co-solvents prior to addition to the binder to improve miscibility. An alternative is using hydrophilically modified polyisocyanates.
Secondly, while in solventborne coatings the solvent is chosen to be inert to the crosslinker, an isocyanate group can readily hydrolyse in waterborne coatings.
Even though in general the rate constants of the reaction between aliphatic polyisocyanates and water are lower than with primary alcohols , the shear excess of water compared to polymer bound hydroxyl groups can cause significant hydrolysis.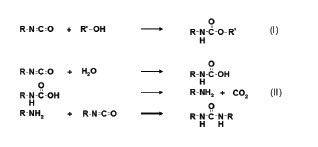
Figure 1. The desired reaction in 2 component polyisocyanate curing coatings (I) and reaction occurring upon hydrolysis of isocyanate (II).
The disadvantage of polyisocyanates hydrolyzing is twofold. When an isocyanate group reacts with water an unstable carbamic acid group is formed, which readily dissociates to form an amine group and carbondioxide. The amine group will react with a second isocyanate group to form a urea bond (see Figure 1).
Hence, hydrolysis does lead to crosslinking, but at the expense of a second crosslinking group. For this reason, waterborne 2C NCO crosslinking coatings are always formulated at an excess of isocyanate compared to hydroxyl groups.
The carbondioxide formed is an issue especially in thick films – i.e. at a dry film thickness of more than 50 μm – where blisters are formed that affect both aesthetics and coherence of the film.
Secondly, although reaction of the amine group with an isocyanate still results in crosslinking, two isocyanate groups are required to achieve this when one of them is first hydrolyzed. As a result, the efficiency of the crosslinker is considerably affected.
Preparation of Binders, Formulations, and Films
In this paper we will discuss film properties of films based on solvent free, hydroxyl functional (meth)acrylate copolymer emulsions with different OH-contents.
A series of polymers containing 2,3,4 and 5% OH (corresponding to OH numbers of 67, 100, 135, and 165 mg KOH/g resin, respectively) were prepared via the secondary emulsion route, i.e. polymerization in an organic solvent followed by neutralization of the acid groups by a base and finally a dispersing step.
After dispersing the polymer in water, the solvent was removed by means of vacuum distillation and a dispersion with less than 1000 ppm solvent at 40% solid content was obtained. Because of practical limitations during polymerization in solvent, the molecular weight of these kind of binders is typically in the 20-50 kD range.
The low molecular weight allows for a good flow of the polymer chains during film formation. Binders based on this technique therefore typically show very good gloss levels after drying.
Also, due to the good film formation,the (water-)barrier properties of such products can also be very good compared to a resin synthesized via normal emulsion polymerization.
Another aspect that helps generating good barrier properties is the absence of (hydrophilic) surfactants.
Surfactants that are needed to stabilize particles during emulsion polymerization processes, may result in high water sensitivity of the film as they remain in the film after drying, and even prevent the formation of a fully intact film .
In the formulation, a hydrophilically modified NCO type (Bayhydur® 3100) was added to these secondary emulsions, in such an amount that the theoretical NCO:OH-ratio was 1, and the solid content of the mixture was corrected to 40%.
This mixture was left to stir for 10 minutes, and applied to the test-substrate after 1 h. The films were all applied by means of a wired rod at a wet film thickness of 100 μm and dried for 4 h at RT before drying at elevated temperature (50°C) for 16 h to yield dry films with a thickness of ~40 μm.
Drying, crosslinking and film formation
In this paper we discuss the film properties of different binders with varying OH-content, ranging from 2% to 5% OH on solid polymer.
Since the NCO-functional crosslinker is also known to plasticize the film during film formation, the typical drying times before mechanical properties are reached, increase with increasing NCO content (and thus with increasing OHnumber).
As was shown in previous work , drying times of WB 2C NCO curing binders can be controlled by the choice of cosolvent. It was shown that when adding solvents with a high evaporation rate, such as Dowanol PM, both the tack free and dust free times can even be reduced compared to a system that is applied without any solvent.
Furthermore, it was shown that for slow evaporating solvents traces of solvent can be found after long drying times (days, or even weeks) and that drying times were significantly increased compared to non-solvent containing binders.
In the present work, the influence of the cosolvent on application properties is avoided by formulating all binders without solvent.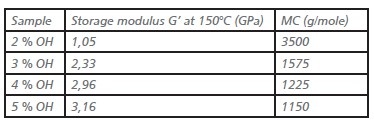
Table 1. Storage moduli and molecular weight between crosslinks (MC) of films containing binders with different hydroxyl numbers.
Dust-free times for the different OH-numbers did not differ significantly, all being between 15 and 20 minutes. This was to be expected as the dust free time is believed to be strongly related to the evaporation rate of water only, which was similar for all binders.
The tack free time did increase significantly with increasing OH-number and, hence, isocyanate concentration in the drying film. This is explained by the higher amount of low viscous isocyanate that is used to crosslink these systems.
The final hardness that was reached at full cure increased as function of the OH-content, because of the increasing crosslink density and the increasing concentration of urethane bonds in the cured films.
Crosslinking levels of the films was confirmed with DMTA measurements. By measuring the storage modulus in the rubber plateau, the molecular weight between crosslinks was calculated.
As can be expected higher hydroxyl contents in the binder lead to higher storage moduli in the rubbery state, and hence a lower molecular weight between crosslinks.
The effect of increasing the hydroxyl content from 4% to 5% seems to be limited, which indicates a less efficient crosslinking and hence more side reactions. More details on the crosslinking efficiency can be found in a separate publication.
Film Formation
Film formation was studied in more detail by means of atomic force microscopy (AFM). As can be seen in Figure 2 the film formation for this type of binders is very good, as was expected due to the limited molecular weight of the acrylic polymers (MW<30 kD).
The height differences on the z-axis are extremely small (<6 nm) indicating that indeed very smooth films are obtained. Also in the phase image a homogeneous picture was observed without any sign of the original particles.
This teaches that film formation was not hindered by vitrification due to crosslinking and suggests that crosslinking occurs homogeneously throughout the films.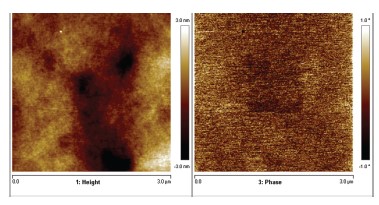
Figure 2. Film formation studied by AFM in height and phase
contrast of a 5 % OH acrylate copolymer.
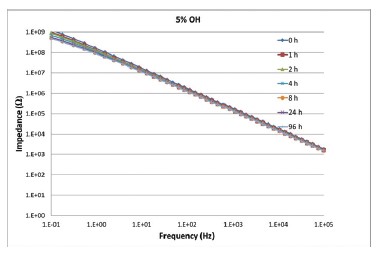
Figure 3. Impedance of a crosslinked film of a 5% OH containing
binder as function of exposure time to 0.1 M Na2SO4 solution as
determined with EIS.
Next to AFM, electrochemical impedance spectroscopy (EIS) was used to obtain information on the extend of film formation and the barrier properties against water ingress. In case of poor film formation (e.g. pinholes), the measured barrier against electrical current would be poor, while for homogeneous intact films a significant impedance will be measured.
As can be seen in Figure 3 the impedance does not significantly change as function of time, indicating a very good barrier and hence homogeneous film without defects.
The change in impedance upon exposure to water can be attributed to the uptake of water by the film. This will result in an increase of the conductivity of the inherently poorly conductive polymeric film. By plotting impedance at high frequency (105 Hz) as function of time, an indication of water uptake can be obtained.
Figure 4 shows that an increase in OH number in the acrylate binder resulted in a lower equilibrium amount of water in the film.
This can be explained by the higher degree of crosslinking. If no crosslinking would take place, an opposite trend would be expected, since with increasing OH number more hydrophilic groups are present in the polymer. These hydrophilic moieties would cause a higher equilibrium water absorption.
Furthermore, the differences between the 4% and 5% OH containing films are relatively small, indicating that the barrier properties do not further improve upon higher crosslink densities.
This is in line with the DMTA results, presented in Table 2, where it was shown that crosslink density did not significantly increase when increasing the OH content from 4% to 5%.
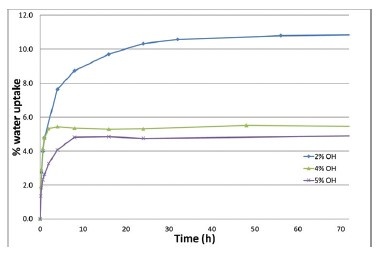
Figure 4. Water uptake profiles as derived by electrochemical impedance spectroscopy from the different OH-containing binders.
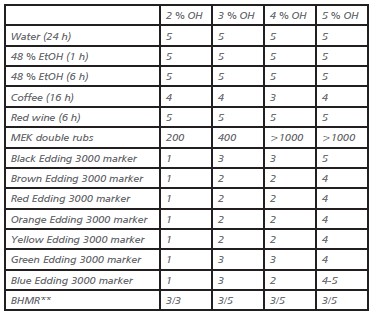
Table 2. Overview of chemical resistances of polymers with different levels of OH.
* Stain resistances are ranked from 0 to 5, where 5 indicates no visible damage, discoloration or gloss loss.
** BHMR was determined before and after removal with a cloth.
Resistances of WB 2C NCO Curing Binders
The good barrier properties of these binders against water ingress suggest that the resistance against stains of water containing chemicals should also be good. The stain resistance against water, coffee, red wine, and ethanol (48% in water) was found to be very good to excellent, even for the low OH containing binders.
The resistance against organic solvents was tested by performing MEK double rubs, where the number of rubs that is needed to remove the coating from the substrate was used as a measure. Here a clear trend was seen: 1000 double rubs were reached without any visible damage of the films for the binders with 4% OH and higher.
A similar trend was seen for different marker pens. A higher OH-content gave a better resistance against stains by these markers. For the 2% containing binder, all individual colors could be clearly identified after cleaning with iso-propylalcohol.
For the 3% and 4% OH binders all colors were still visible, but the intensity was significantly less compared to the 2% OH binder.
For the binder containing 5% of hydroxyl groups only a shadow of the colors could be seen, with the blue color nearly completely and the black color being completely removed. This shows that for passing this test very high crosslinking levels are required.
The black heel mark resistance was determined by striking a coated Leneta test chard with a standard heel with a high percentage of carbon black and hit the coating with the heel.
If no black marking is found on the coating, the rating is a 5, while in case of a heavy black mark a 0 rating is given. The test results given in Table 3 on BHMR are presented before and after manually removing the black mark by cleaning with a cloth.
As can be expected, the 2% OH -containing film showed a poorer black heel mark resistance compared to the higher OH numbers. Furthermore, it showed that the 3% OH based film had a similar score as the 4% and 5% OH containing ones.
This suggests that a hydroxyl content of 3% should be sufficient to obtain films with sufficient mechanical properties to not be damaged in a black heel mark resistance test.
2C NCO Curing Binders for Flooring Applications
Although the mechanical properties and chemical resistances of 2C NCO curing acrylic resins are very good compared to 1C acrylic resins, their use in flooring applications until now is limited.
One of the drawbacks of waterborne 2C systems is the development of CO2 blisters in thicker films (dft >100-200 microns) , which is caused by hydrolysis side reactions. This risk of blister formation naturally increases with increasing OH-number.
Furthermore, for the professional high wear market (e.g. barber shops, high traffic shopping areas, garage floors) mechanical properties of acrylate based 2C NCO curing films are often not good enough.
The main shortcoming of acrylic binders is that upon high friction, the heat development that is developed by the friction can cause plastic deformation, even in a highly crosslinked film! To overcome these issues, blending of the acrylic polyol emulsion with an additional binder can be a solution.
In this paper we will focus on blending water based polyurethane resins with a 5% OH containing 2C NCO curing binder (NeoCryl® XK- 555) to optimize performance of the combined product. Urethane emulsions used in the work for this paper were NeoRez® R-2180 and NeoRez® R-1010.
The crosslinker used for this work was Bayhydur® XP 2700, which is a 65:35 mixture of a hydrophilically modified polyisocyanate and Dipropylene Glycol Dimethyl Ether (DMM). In the formulation, this crosslinker can only react with NeoCryl® XK-555, since the two urethane emulsions are not hydroxyl functional.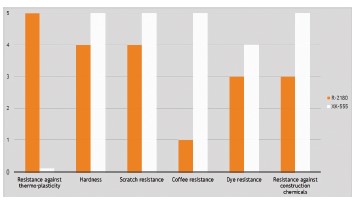
Figure 5. Overview of film properties of the hydroxyl functional WB 2C NCO curing binder (NeoCryl® XK-555) formulated with 20% wtof Bayhydur® XP 2700 (a 65:35 mixture of a hydrophilically modified polyisocyanate and DMM), and a waterborne polyurethane binder (NeoRez® R-2180). Properties are rated on a relative scale (0-5) with a 5 giving excellent properties and a 0 indicating poor performance.
As shown in Figure 5, the resistance against thermoplastic deformation of the urethane emulsion significantly exceeds that of the acrylic 2C film. On the other hand, the resistances of the WB 2C product are significantly better than these of the WB urethane.
To make optimal use of the individual properties of the 2C NCO curing binder and the polyurethane, different blend ratios were investigated.
Figure 6 shows that blending 30wt-% of the polyurethane product with the 5% OH containing acrylic emulsion gave an optimal combination of film properties, all rated on a relative scale of 0-5, with 5 being excellent and 0 being poor.
The thermoplastic behavior of the blend increased significantly, without compromising too much on mechanical properties, such as hardness or scratch resistance, or chemical resistances such as against dye, coffee, or construction chemicals.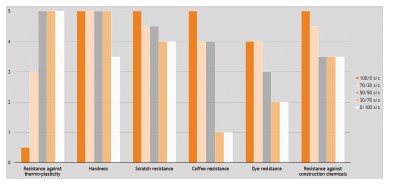
Figure 6. Film properties of blends of NeoCryl® XK-555 and NeoRez® R-2180 as function of blend ratio; crosslinked with 20 wt-% (on XK-555 dry weight) of
Bayhydur® XP 2700.
Next to high wear applications, also matt finishes are a field where waterborne 2C NCO curing acrylic resins can add a great deal to improve the performance. Matt finishes are a stylistic choice for flooring applications to keep a natural look to a wooden floor.
One product that can be used to obtain these matt properties is NeoRez® R-1010, a water based polyurethane emulsion. However, coatings based on this product are lacking sufficient hardness and scratch resistance as required in flooring applications.
By blending this product in a 75/25 ratio with NeoCryl® XK-555, an excellent balance between appearance and performance can be obtained.
Mechanical resistances such as scratch and black heel mark are significantly improved, and also the chemical resistances against house hold chemicals and disinfectants show a strong improvement, almost to the level of the pure 2C NCO film, as is shown in Figure 7.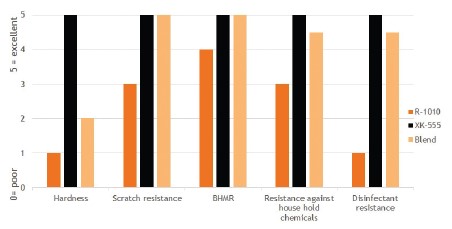
Figure 7. Film properties of a pure polyurethane product (NeoRez® R-1010) and a 2C 5% OH containing polyacrylate (NeoCryl® XK-555) compared to the
performance of a 75/25 blend of these products.
Even though the 2C NCO curing polyacrylate has a very high gloss level due to the high extend of film formation, an ultra-matt appearance can effectively be reached for the blend described above.
Upon addition of only a few percent of matting powders, the gloss level at 850C can be reduced to below 10%. This shows that 2C NCO curing acrylates can be used to improve the film performance of non-functional polyurethane binders to a level that makes the combination very suitable as parquet top coat.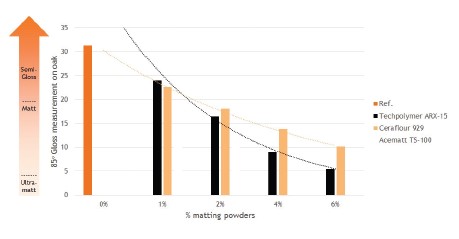
Figure 8. Reduction of the 85°C gloss level by addition of matting powders for a NeoRez® R-1010: NeoCryl® XK-555 blend in a 75:25 ratio. The blend
was formulated with 10% of Easaqua XL-600/ PC (80/20) on binder
Conclusions
2C NCO curing water based polyacrylate resins can give extremely good properties in terms of (stain) resistances, caused by their high degree of film formation in combination with a high crosslink density.
Due to their high degree of crosslinking, these products as such are not useful for high wear flooring applications, as they lack thermoplastic behavior.
When blending these products with water based polyurethane binders, excellent flooring application properties can be obtained, combining excellent resistance profiles with good mechanical properties. Both on high-wear and aesthetics, formulations can be made to obtain a perfect fit for either application.

Willem Jan Soer
Scientist Acrylic Emulsions
DSM Coating Resins B.V

Çiğdem Roman
Coatings, Inks, Adhesives Product Manager
IMCD Turkey
References
1. Tijs Nabuurs, Akrilik Emülsiyonlar Kıdemli Bilim İnsanı / Senior Scientist Acrylic Emulsions , DSM Coating Resins B.V
2. Sjoerd Buil, Zemin Kaplamaları ve Yapı Bölümü Global Endüstri Müdürü / Global Industry Manager Flooring and Construction , DSM Coating Resins B.V
3. H. Bui, M. Dvorchak, K. Hudson, J. Hunter; Eur. Coat. J. 97 (1997) 476
4. M. Melchiors, M. Sonntag, C. Kobusch, E. Jürgens; Prog. Org. Coat. 40 (2000) 99
5. Z. Wicks, D. Wicks, J. Rosthauser; Prog. Org. Coat. 44 (2002) 161
6. Liu, Y., Gajewicz, A. M., Rodin, V., Soer, W.-J., Scheerder, J., Satgurunathan, G., McDonald, P. J. and Keddie, J. L. (2016), J. Polym. Sci.Part B: Polym. Phys., 54: 1658–1674.
7. T. Nabuurs, W.J. Soer, W. van Bavel, J. vd Werf, Eur. Coat. J. 10 (2009) 28
8. L. Hill; Prog. Org. Coat. 31, p. 235 (1997)
9. T. Nabuurs, W.J. Soer, R. Peters, polymer international, submitted.
10. V. D. M. Brasher, A. H. Kingsbury, J. Appl. Chem. 4 (1954) 62.
11. T. Nabuurs, D. Pears, A. Overbeek; Prog. Org. Coat. 35 (1999) 129





