Introduction
With the developing technology and increasing consumption, there is an increase in the use of electrical and electronic equipment. It leads to an increase in battery usage in general. The batteries vary according to the device and sector they are used in, and they are divided into two types primary (non-rechargeable) batteries and secondary (rechargeable) batteries in terms of working principle. While the reactions in primary batteries are irreversible, the reactions in secondary batteries can be reversed by supplying electrical energy from the external. With the increase in the use of disposable batteries, the problem of waste batteries arises and at the same time, the amount of waste generated is increasing due to the wide use of lithium-ion batteries, which are a type of rechargeable battery.
The most common types of lithium-ion batteries are lithium cobalt oxide (LiCoO2), lithium manganese oxide (LiMnO2), lithium nickel cobalt manganese (LiNiCoMnO2), lithium
iron phosphate (LiFePO4), lithium nickel cobalt aluminum (LiNiCoAlO2), and lithium titanate (Li4Ti5O12).
In addition to graphite, cobalt, and lithium, lithium-ion batteries contain aluminum, nickel, manganese, copper, titanium, some rare earth elements, and plastics. The recovery of these elements, known as critical and strategic raw materials, has great importance. The recovery of precious metals from lithium-ion batteries become virtual in terms of economic reasons, developing technology, and the increasing demand for some critical raw materials.
Lithium-ion batteries generally consist of three parts: anode, cathode, and electrolyte. The cathode includes metal oxides and its most widely used commercial type is LiCoO2. The anode consists of a porous carbon structure and the main material used for this purpose is graphite. The electrolyte, on the other hand, consists of organic solvents such as LiPF6, which generally contains lithium ions (Vıcıl, 2011). Figure 1 shows the parts of the battery and their material contents (Ellingsen and Hung, 2018).
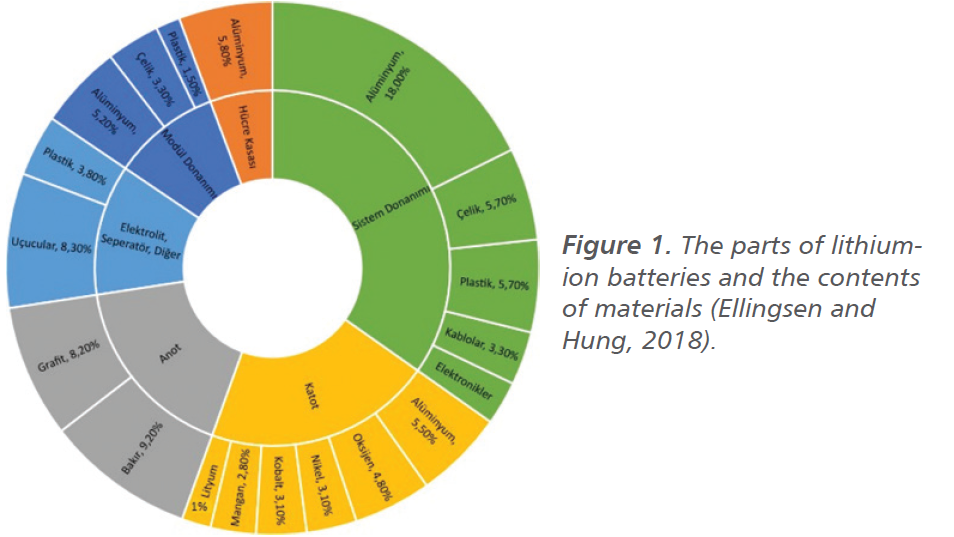
Lithium-ion battery technology is developing day by day. With these developments, the usage areas are increasing and therefore the amount of usage shows parallelism. Lithium-ion batteries, which have become widespread with the use of mobile phones and laptops,
have started to be used in solar energy storage systems in much larger capacities, in electrical vehicles, and in buses. Their use in almost many technological devices has greatly increased the volume of the lithium-ion battery market (Pillot, 2019).
Lithium-ion batteries may cause dangerous situations such as burning and flashing when exposed to high heat. For this reason, many studies and technologies have been developed on the safe use of lithium-ion batteries from past to present. Especially in technological devices that contain large amounts of batteries, such as electrical vehicles, have cooling systems. Devices that do not have a cooling system, such as mobile phones, should not be exposed to the sun directly. New technology in lithium-ion batteries provides a much safer use than their previous types. Thus, lithium-ion batteries can be easily used in electrical vehicles. In the meantime, they are preferred due to their low cost and simple maintenance. There are a variety of advantages of lithium-ion batteries due to their long-lasting and longterm storability (Prabhakar, 2008).
Lithium cobalt oxide (LCO) type lithium-ion batteries have a high specific energy. Having high specific energy means that the ratio of the amount of energy it stores to the battery volume is quite high. Therefore, LCO type of batteries is mostly used in portable technological devices such as mobile phones and laptops (Akkuş, 2011).
Lithium-ion batteries mainly consist of graphite on the copper anode and lithium cobalt oxide on the aluminum cathode. In the case of functioning, lithium ions move from the anode to the cathode, this electrochemical reaction occurs in the opposite direction of charging (Şahan and Patat, 2014). Despite their high specific energy, LCO type batteries have a low cycle count. Therefore, they have to be changed frequently. Although the type of lithium-ion battery varies according to the area of use, they are used as small hand tools, electrical vehicles, and high energy storage sources. The structure and parts of lithium-ion batteries used in mobile phones are shown in Figure 2 and the anodic and cathodic reactions in LCO batteries are shown in reactions 1 and 2 (Gülbeyaz, 2019).
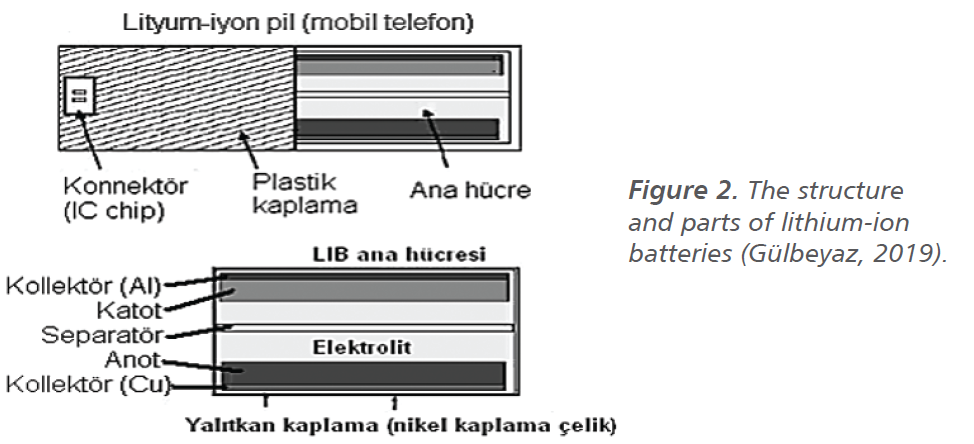
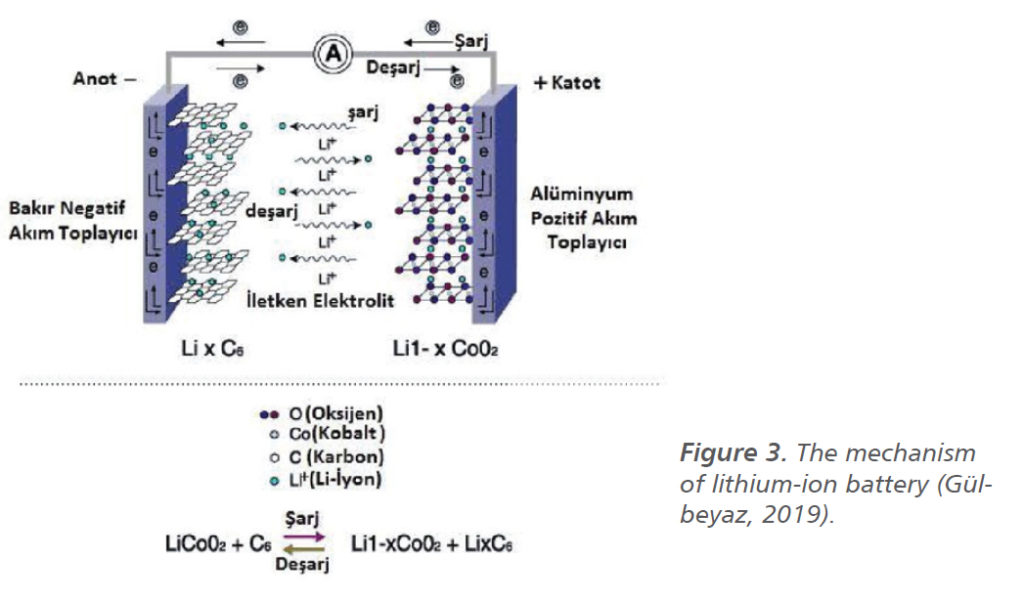
Chemical changes occur in the active cathode material to a great extent. This change, which creates different types of lithium-ion batteries, directly affects the need for use. Thanks to the different raw materials used, capacity, power, and lifetime increases can be achieved in lithium-ion batteries (Miao et al., 2019). The anode and cathode can be called electrodes in general, and these electrodes have a layered structure, and these structures can also be spinel structures. Because of its layered structure, the cathode provides charge-discharge of the cell. During charging and discharging, lithium ions are exchanged between the positive and negative electrodes, and the displacement reactions of lithium ions are called topotactic (Bolat et al., 2012). The anode and cathode are active materials in the chemical reaction in the battery. As it is seen in Figure 3, lithium switches between active materials (Şahan and Patat, 2014; Gülbeyaz, 2019).
According to the reports, it was mentioned that the market volume of lithium-ion batteries has reached 1 billion dollars in 2017, is predicted that it will reach 36 billion dollars in 2020 and 75 billion dollars in 2030 (Pillot, 2019). Lithium-ion batteries, which were used only in devices such as laptops and mobile phones at the beginning of the 2000s, have reached very high gigawatt values in the 2020s due to their use in higher technology areas such as electric vehicles. The change in the total amount of spent lithium-ion batteries over the past ten years is shown in Figure 4 (Melin, 2020).
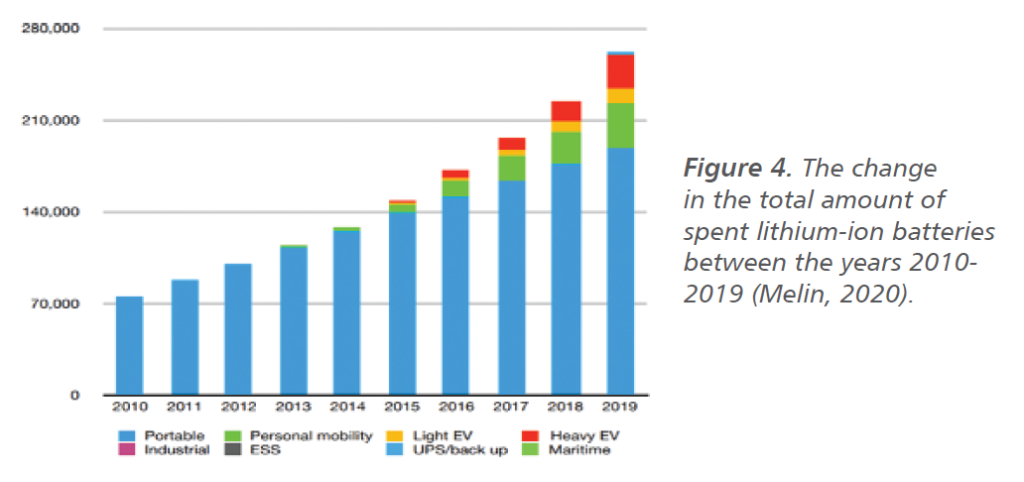
The volume, which was 1000 gigawatts/hour in 2020, is expected to increase approximately three times towards 2025. According to analytical reports, this figure is expected to increase 11 times in 2030 with the rapid increase in the number of electric vehicles (Melin, 2020).





