Acid pickling is a treatment of metallic surfaces to remove impurities such as rust or scale with a solution called pickle liquor which are mainly strong mineral acids. The two acids commonly used are hydrochloric acid and sulfuric acid. Phosphoric, hydrofluoric, or nitric acids are also used.
Sulfuric or hydrochloric acids are used for pickling of carbon steels, and phosphoric, nitric, and hydrofluoric acids are used together with Sulfuric acid for stainless steel. Acid pickling operation can be a batch or continuous process depending on the product being pickled.
In this paper we are going to focus on recovery of sulfuric acid or hydrochloric acid from waste pickling liquors.
Chemical reactions:
Impurities on the steel surface can be iron oxides like FeO, Fe2O3 and Fe3O4. Mainly, following reactions take place.
Pickling with Sulfuric Acid:

The ferrous sulfate that is formed in the above reaction is either monohydrate or heptahydrate depending on the crystallization temperature.
Pickling with hydrochloric acid:

An inhibitor is usually added to decrease the acid’s attack on the base metal while allowing it to act on the iron oxides.
The rate of pickling increases with the increase of temperature and concentration of acid as seen in Fig.1 and Fig.2. Increase in FeCl2 concentration up to some level also increases
the pickling rate (Fig. 3). As pickling continues, acid is depleted and iron compounds build up in the pickling liquor to a point where pickling is no longer effective. The depleted liquor is discharged and the pickling tank is refilled with fresh acid. Possible solutions to waste pickling liquors are listed below;
1. Offsite disposal,
2. Onsite neutralization/clarification and disposal to waste water treatment plants,
3. Recovery of the acid.
Option 1&2 has following disadvantages,
• High cost of offsite disposal and neutralization/clarification,
• Loss of acid by disposal. So, fresh acid requirement will
increase,
• Discharging and refilling of the acid tanks will cause loss
of time and production,
• Variable acid and iron concentration in the pickling
tanks will effect the pickling time and quality,
• These methods are not environment-friendly.
 Figure 1
Figure 1. Pickling time for sulfuric acid. [2]
Figure 2. Pickling time for hydrochloric acid. [2]

Figure 3. Effect of FeCl2 concentration on pickling times at 20°C. [2]
Advantages of acid recovery:
• It is environment-friendly.
• There is no cost of disposal or neutralization/clarification.
• There is no loss of acid by disposal. So, fresh acid requirement will decrease.
• Closed loop system will increase the efficiency of the production.
• Optimum and constant acid/iron concentrations in the pickling tanks will increase the pickling rate and quality.
• Recovered water in HCl recovery system will be reused in the process.
• Saleable by-products like ferrous sulfate heptahydrate, ferrous chloride can be obtained.
• Easy operation and installation of these modular recovery systems.

Figure 4. Acid Recovery Systems
Acid solution from the pickling tanks is fed to the recovery system. By-products like ferrous sulfate heptahydrate and ferrous chloride are obtained through the recovery process. Recovered acid is sent back to the pickling tanks. Make-up acid is added to the system according to the pickling reactions mentioned above.
In Sulfuric acid recovery system, water is leaving the system as heptahydrate with Ferrous Sulfate. So, water should be added to the system according to the overall mass balance. Rinse water can be used for this purpose. In Hydrochloric acid recovery system, water from condenser can be reused in the process such as rinse water.
1.0 Recovery of Sulfuric Acid by Cooling Crystallization
Pickling time decreases as temperature increases for sulfuric acid (Fig. 1). Most pickling tanks are operated between 45oC and 60oC with a Sulfuric acid concentration of 15-20%. If that solution is cooled down to around 10-15oC, most of the ferrous sulfate will come out of the solution as ferrous sulfate heptahydrate crystals.
Solubility of ferrous sulfate in sulfuric acid can be seen in Fig. 5. Iron concentration will be around 55-65 g/L Fe in pickling tanks. Process can be described as follows (Fig. 6);
1. After filtering the acid solution, it is cooled down through the economizer before the crystallization. It is cooled with the solution that is coming out from the crystallization system.
While temperature of the recovered acid is increased through the economizer, temperature of the feed solution which enters to the crystallization system is decreased. Thus, required energy for heating and cooling is minimized.
2. Cooled acid solution is fed to the crystallization system. Further cooling in the crystallizer will give Ferrous Sulfate Heptahydrate crystals (FeSO4.7H2O) because of the supersaturation created. Heat exchanger should be carefully designed in order to prevent crust formation.
3. Since the acid recovery process is a closed loop continuous system, optimum and constant acid/iron concentrations can be maintained in the pickling tanks.
4. The by-product ferrous sulfate heptahydrate (FeSO4.7H2O) which has a commercial value can be used in agricultural sector or waste water treatment plants.

Figure 5. Ferrous Sulfate in Aqueous Sulfuric Acid. [3]
As mentioned before, water is leaving the system as heptahydrate with ferrous sulfate. So, water should be added to the system according to the overall mass balance. Since the operated media is very corrosive because of the operation temperature and acid content, material of construction should be selected carefully.
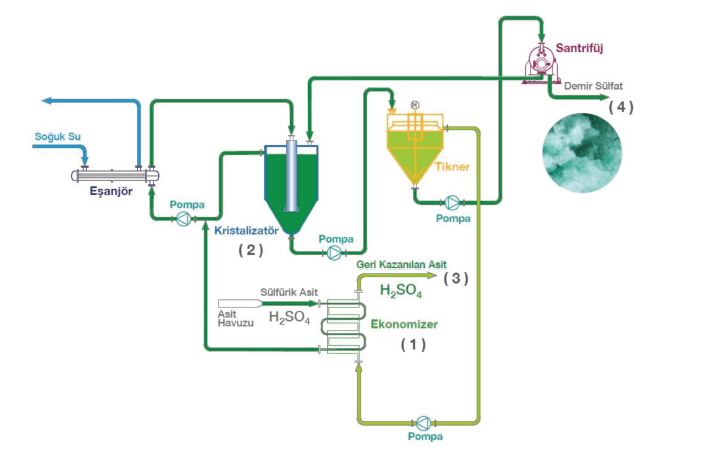
Figure 6. Recovery of Sulfuric Acid by Cooling Crystallization

Figure 7. Modular Design of Sulfuric Acid Recovery Plant
2.0 Evaporative Recovery of Hydrochloric Acid
Recovery of hydrochloric acid from pickling liquors is different than sulfuric acid. The process can be describes as follows (see Fig. 8);
1. After filtering the acid solution, it is fed to the evaporator. Through the evaporation of acid and water, concentration of Ferrous Chloride increases in the liquid phase. Further evaporation will produce Ferrous Chloride Dihydrate (FeCl2.2H2O) crystals.
2. Acid and water vapor from the evaporator come to the rectification column. It is a kind of distillation column. Water vapor enriches in it. Hydrochloric acid is the bottom product while water is the top product. Purities can be adjusted by adjusting the reflux ratio. Because of the azeotrope between HCl and water, concentration of HCl can reach up to 18% (w/w).
3. Water vapor is condensed in the condenser and sent to the process for reuse.
4. Recovered acid is reused in the pickling tanks.
5. Ferrous chloride has commercial value so, it can be used in waste water treatment plants. It is used to control odor problems in sewers. It reduces H2S odors to a harmless iron compound, controlling odors and lessening corrosion.

3.0 Economic Evaluation of the Acid Recovery System
Case Study: A plant which has processing capacity of 150 ton/day steel is analyzed both considering the offsite disposal and evaporative acid recovery system.
Accepted Values:
Cost of offsite disposal: 50.42 USD/ton
Cost of fresh HCl (32%): 102.5 USD/ton
Cost of Electricity: 0.083 USD/kWh
Cost of Natural Gas: 0.3227 USD/Nm3
Operation hours: 7035 h/year
FeCl2.2H2O selling price: 100 USD/ton
Utilities:
Power consumption: 15 kW
Natural gas consumption: 32.25 Nm3/h
Table 1 Comparison of costs for two methods

If acid recovery process is used;
Annual return:
307,936 – 169,073 = 138,863 USD/year
Income from by-product sales:
61,200 USD/year
Total annual return:
138,863 + 61,200 = 200,063 USD/year
Investment Cost:
500,000 USD
Payback Period:
(500,000 USD)/(200,063 USD)≈2.5 year
 Şaban Kantaşlı
Şaban Kantaşlı
Chemical Engineer (M.Sc.)
Project Development Manager
Sistemas Teknoloji A.Ş.
References
[1] Lawrence K. Wang, Yung-Tse Hung, Nazih K. Shammas, Handbook of Advanced Industrial and Hazardous Wastes Treatment, CRC Press 2010.
[2] Mika Maanonen, Steel Pickling in Challenging Conditions, Thesis 2014.
[3] J.K. Seyler, W.E. Thornton, M.K. Householder, Sulfuric Acid and Ferrous Sulfate Recovery from Waste Pickle Liquor, EPA Jan 1974.
[4] T. Özdemir, C. Öztin, N. S. Kıncal, Treatment of Waste Pickling Liquors: Process Synthesis and Economic Analysis
[5] J. Cullivan, B. Cullivan, Economic and Chemical Comparisons of Hydrochloric Acid Recovery Technologies for Iron Pickling Operations, Wire Journal
International, March 2016.

 The ferrous sulfate that is formed in the above reaction is either monohydrate or heptahydrate depending on the crystallization temperature.
The ferrous sulfate that is formed in the above reaction is either monohydrate or heptahydrate depending on the crystallization temperature.
 An inhibitor is usually added to decrease the acid’s attack on the base metal while allowing it to act on the iron oxides.
The rate of pickling increases with the increase of temperature and concentration of acid as seen in Fig.1 and Fig.2. Increase in FeCl2 concentration up to some level also increases
the pickling rate (Fig. 3). As pickling continues, acid is depleted and iron compounds build up in the pickling liquor to a point where pickling is no longer effective. The depleted liquor is discharged and the pickling tank is refilled with fresh acid. Possible solutions to waste pickling liquors are listed below;
1. Offsite disposal,
2. Onsite neutralization/clarification and disposal to waste water treatment plants,
3. Recovery of the acid.
An inhibitor is usually added to decrease the acid’s attack on the base metal while allowing it to act on the iron oxides.
The rate of pickling increases with the increase of temperature and concentration of acid as seen in Fig.1 and Fig.2. Increase in FeCl2 concentration up to some level also increases
the pickling rate (Fig. 3). As pickling continues, acid is depleted and iron compounds build up in the pickling liquor to a point where pickling is no longer effective. The depleted liquor is discharged and the pickling tank is refilled with fresh acid. Possible solutions to waste pickling liquors are listed below;
1. Offsite disposal,
2. Onsite neutralization/clarification and disposal to waste water treatment plants,
3. Recovery of the acid.
 Figure 1. Pickling time for sulfuric acid. [2] Figure 2. Pickling time for hydrochloric acid. [2]
Figure 1. Pickling time for sulfuric acid. [2] Figure 2. Pickling time for hydrochloric acid. [2]






 If acid recovery process is used;
Annual return:
307,936 – 169,073 = 138,863 USD/year
Income from by-product sales:
61,200 USD/year
Total annual return:
138,863 + 61,200 = 200,063 USD/year
Investment Cost:
500,000 USD
Payback Period:
(500,000 USD)/(200,063 USD)≈2.5 year
If acid recovery process is used;
Annual return:
307,936 – 169,073 = 138,863 USD/year
Income from by-product sales:
61,200 USD/year
Total annual return:
138,863 + 61,200 = 200,063 USD/year
Investment Cost:
500,000 USD
Payback Period:
(500,000 USD)/(200,063 USD)≈2.5 year
 Şaban Kantaşlı
Chemical Engineer (M.Sc.)
Project Development Manager
Sistemas Teknoloji A.Ş.
References
[1] Lawrence K. Wang, Yung-Tse Hung, Nazih K. Shammas, Handbook of Advanced Industrial and Hazardous Wastes Treatment, CRC Press 2010.
[2] Mika Maanonen, Steel Pickling in Challenging Conditions, Thesis 2014.
[3] J.K. Seyler, W.E. Thornton, M.K. Householder, Sulfuric Acid and Ferrous Sulfate Recovery from Waste Pickle Liquor, EPA Jan 1974.
[4] T. Özdemir, C. Öztin, N. S. Kıncal, Treatment of Waste Pickling Liquors: Process Synthesis and Economic Analysis
[5] J. Cullivan, B. Cullivan, Economic and Chemical Comparisons of Hydrochloric Acid Recovery Technologies for Iron Pickling Operations, Wire Journal
International, March 2016.
Şaban Kantaşlı
Chemical Engineer (M.Sc.)
Project Development Manager
Sistemas Teknoloji A.Ş.
References
[1] Lawrence K. Wang, Yung-Tse Hung, Nazih K. Shammas, Handbook of Advanced Industrial and Hazardous Wastes Treatment, CRC Press 2010.
[2] Mika Maanonen, Steel Pickling in Challenging Conditions, Thesis 2014.
[3] J.K. Seyler, W.E. Thornton, M.K. Householder, Sulfuric Acid and Ferrous Sulfate Recovery from Waste Pickle Liquor, EPA Jan 1974.
[4] T. Özdemir, C. Öztin, N. S. Kıncal, Treatment of Waste Pickling Liquors: Process Synthesis and Economic Analysis
[5] J. Cullivan, B. Cullivan, Economic and Chemical Comparisons of Hydrochloric Acid Recovery Technologies for Iron Pickling Operations, Wire Journal
International, March 2016.