Introduction
An organic coating system protects metal substrates against corrosion by means of one or more of several mechanisms:
• Decreasing the rates of anodic (oxidation) and/or cathodic (reduction) corrosion half-reactions that occur on the metal/paint interface.
• Introduction of high electrical resistance into the circuit of the metal/electrolyte corrosion cell that is caused by the nonconductive organic film that is applied on the metal substrate.
• Acting as an effective physical barrier against the trans-port of aggressive species, such as water, which is the basis of the electrolyte/humidity conditions; oxygen, which is the oxidizing agent; and various corrosion ions (Cl–, SO4–2, etc.) to the metal surface.
In general, the anti-corrosion efficiency of an organic primer film is a function of four factors:
1. The nature of the metal substrate, that is, its susceptibility to oxidative corrosion.
2. The state of the metal/primer interface—for example, its electrical resistance and capacity, the nature of ions or molecules present in this region, etc.
3. The composition of the primer film and efficiency of the anti-corrosion pigments and any other inhibitors.
4. Corrosion aggressivity of the environment and the characteristic nature to which the coated metal is exposed.
Metal corrosion and the relevant electrochemical theory are described elsewhere. This chapter deals with the protection of metal surfaces against corrosion by means of coatings that contain anticorrosive pigments.
The degree of corrosion protection depends not only on the pigment, but also on the binder, and these must complement each other chemically. Anticorrosive pigments may be divided into three types:
1) Pigments with a Physical Protective Action:
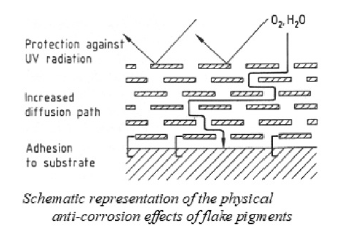
Chemically inert and are termed inactive or passive. An example is micaceous iron ore. These lamellar pigments are packed in layers; they lengthen the path ways and obstruct the penetration of ions. They improve adhesion between the substrate and the coating, absorb UV radiation, and protect the underlying binder.
2) Pigments with Chemical Protective Action:
Contain soluble components and can maintain a constant pH value in the coating. They are termed active and their action depends on reactions in the interfacial areas between the pigment and substrate, pigment and binder, or between pigment and ions that penetrate into the coating.
An example is red lead. Redox reactions can occur to form protective compounds (oxides or oxide hydrates that may contain pigment cations). Saponification of the binder or neutralization of the acidic decomposition products enable a defınite pH value to be maintained in the coating.
3) Pigments with An Electrochemical Protective Action:
Passivate the metallic surface. Those that prevent corrosion of the iron by forming a protective coating (e.g., phosphate pigments) are regarded as being active in the anodic region of the metal surface (anodic protection).
Pigments that prevent rust formation due to their high oxidation potential (e.g., chromates) are said to be active in the cathodic region (cathodic protection).
Corrosion of iron is explained by the position of iron in the electrochemical series of the elements (Fe/Fe2+: —0.44 V). In Steel, local anode and cathode areas are found due to the presence of phases containing, for example, carbon, carbides, and oxides.
These latent local cells are activated by moisture, oxygen, and current- carrying electrolytes and the following reactions occur between the anode areas consisting of iron, and the cathode areas containing carbides or oxides. The rust can promote further corrosion.
The OH- ions formed at the cathode produce local high alkalinity and cause hydrolysis of, for example, esterifıed binders, resulting in detachment of the primer from the substrate. Active anticorrosive pigments inhibit one or both of the two electrochemical partial reactions.
The protective action is located at the interface between the substrate and the primer. Water that has diffused into the binder dissolves soluble anticorrosive components (e.g., phosphate, borate, or organic anions) out of the pigments and transports them to the metal surface where they react and stop corrosion.
The oxide film already present on the iron is thereby strengthened and sometimes chemically modified. Any damaged areas are repaired with the aid of the active substance. Inhibition by formation of a protective film is the most important mode of action of the commoner anticorrosive pigments.

Phosphate ions form protective coatings of basic iron (III) phosphate on an iron surface. The reactions of phosphate pigments in binders and on metal surfaces were described.
They postulate that basic complexes are formed from phosphate pigments containing water of crystallization and they react with inorganic ions or with carboxyl groups of the binder. Reaction of the pigment with the binder results in physical changes such as deterioration of adhesion or gelling.
In paints that contain hydroxy- and carboxy-functional binders, gelling during storage can be prevented by using an excess of alcohol as the solvent.
Zinc Phosphate:
The most important phosphate-containing pigment is zinc phosphate, Zn3(PO4)2 · 4H2O. It can be used with a large number of binders and has a very wide range of uses. Zinc phosphate is usually produced on an industrial scale from zinc oxide and phosphoric acid, or from zinc salts and phosphates.
The mechanism of the action of zinc phosphate is shown below. Zinc phosphate di- hydrate pigment is hydrated to the tetrahydrate in an alkyd resin binder. The tetrahydrate is then hydrolyzed to form zinc hydroxide and secondary phosphate ions which form a protective film of basic iron (III) phosphate on the iron surface.
The anticorrosive action of zinc phosphate depends on its particle size distribution. Micronization improves the anticorrosive properties.
Aluminum Phosphate:
Commercial aluminum phosphate anticorrosive pigments consist of aluminum zinc phosphate hydrates, or zinc-containing aluminum triphosphate.
Their composition and properties are in the table below. Aluminum zinc phosphate hydrate pigments are produced by reacting acid Solutions of aluminum hydrogen phosphate with zinc oxide and alkali aluminate. The precipitated pigment is filtered off from the mother liquor, washed, dried, and ground.
Commercial aluminum triphosphate pigments contain ions of trimeric phosphoric acid which form stable aluminum-containing iron phosphate complexes. The aluminum phosphate pigments give good adhesion of the paint film to the metallic substrate.
Composition and properties of aluminum phosphate anticorrosive pigments
Chromium Phosphate:
Chromium phosphate CrPO4·3H2O, is produced from chromium (III) salts and alkali phosphates. Physical and chemical properties are listed in the table below. Chromium phosphate has a low solubility.
It is therefore nearly always used in combination with other anticorrosive pigments. It is an extremely good long-term inhibitor but is less effective during the initial phase of corrosion protection.

New Pigments Based on Metal Phosphates:
Developments in the field of phosphate pigments are aimed at correcting the deficiencies of phosphate (such as the moderate corrosion protection against accelerated weathering in the salt spray test) during the initial phase of corrosion protection.
Improvements include crystallization to form new structures, replacement of cations (e.g., of zinc by calcium), modification of anions (phosphovanadates and phosphocarbonates), optimization of the particle size distribution, providing the zinc ions and phosphate ions in separate compounds, and use of synergistic effects.
Multiphase Phosphate Pigments:
Multiphase anticorrosive phosphate pigments are based on appropriate combinations of inorganic phosphates with sparingly soluble, electrochemically active organic corrosion inhibitors. The multiphase pigments are produced in one combined synthesis.
A slurry of precipitated zinc phosphate is mixed intensively with the zinc or calcium salt of 5-nitroisophthalic acid, fıltered, and washed and ground until the specifıed conductivity is reached. They are more effective than physical mixtures of pigments produced in separate processes.
Multiphase phosphate pigments are used in place of zinc chromate if the anticorrosive effect of pure zinc phosphate is insufficient in the initial weathering phase (e.g., in the salt spray test).
The very good anticorrosive effect of multiphase pigments in air-drying alkyd resins or in alkyd-melamine stoving enamels is comparable to that of zinc chromate, even in open air weathering.
Commercial multiphase pigments differ mainly in the solubility of the organic corrosion inhibitor. Systems with more soluble inhibitors are used in the binders with a low swelling capacity (e.g., epoxy resins or chlorinated rubber).
Other Phosphorus-Containing Pigments
Iron Phosphide: Commercial iron phosphide anticorrosive pigments usually consist of Fe2P, with traces of FeP and SiO2. Iron phosphide anticorrosive pigments are recommended by manufacturers as replacement materials for zinc dust to reduce the price of zinc-rich paints.
Epoxy zinc rich primer or topcoat, inorganic zinc silicate primer or topcoat or used to make corrosion resistant and conductive paint production. A trade name for iron phosphide is Ferrophos.
Zinc Hydroxyphosphite:
Commercial zinc hydroxyphosphite is a white, nontoxic pigment with basic character. As an anticorrosive pigment, zinc hydroxyphosphite is mainly used in air-drying alkyd-based primers, 2-component epoxy primers, chlorine rubber primers and waterborne polymer dispersion paints. The pigment has the following properties.
Borosilicate Pigments:
Borosilicate pigments usually contain calcium or zinc ions in a matrix of Silicon dioxide and boron trioxide, x (Ca,Zn)·y SiO4·zBO3. Aqueous slurries of this white pigment are alkaline. Borosilicate pigments are recommended as nontoxic alternatives to basic lead silicate.
Their main field of application is in waterborne binders and electrodeposition coatings. The properties of the commercial product calcium borosilicate are as follows.
Borate Pigments:
Barium metaborate and zinc borophosphate are colorless pigments. They both have a relatively high-water solubility. Barium metaborate is coated with silica to reduce this. The anticorrosive effects of these pigments depend mainly on their ability to maintain high pH values in the coating.
They are most effective in the initial phase of corrosion protection. The borate ion neutralizes acidic foreign ions and binder decomposition products but is also thought to act as an anodic passivator, forming a protective film. The effectiveness of metal borate pigments in aqueous air-dried anticorrosive coatings is described in.
The metal borate pigments are classifıed as having a relatively low toxicity. As an anticorrosive pigment, barium metaborate is mainly used in air drying alkyd-based primers, alkyd – melamine primers and aqueous polymer dispersion paints.
Zinc borophosphate as anticorrosive pigment is used in air drying alkyd-based primers, 2-Component epoxy primers, aqueous polymer dispersion paints and PVB primers.

M. Namık Kayaalp
Chemical Engineer
Ecelak Boya Kimya San. Tic. Ltd. Şti.





