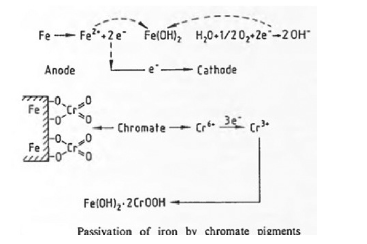
Anticorrosive Chromate Pigments: The anticorrosive action of the chromate pigments is based both on Chemical and electro-chemical reactions, Electrochemical passivation and chemical reaction are illustrated below.
Passivation is based on electrochemical processes in the cathodic region. In addition, a protective film is also formed by reaction of chromate ions with metal ions at the surface of the substrate to form metal oxide hydrates.
The anticorrosive properties of this class of pigments depend on: • The content of water-soluble chromate ions, • The ratio of water-soluble chromate ions to water- soluble corrosion-promoting ions (chloride and sulfate ions), • The active pigment surface in the coating (i.e., particle size distribution and dispersibility).
Chromate-containing pigments are classified as toxic; their use is therefore very limited, and they must be appropriately labeled.
Zinc-Containing Anticorrosive Chromate Pigments Zinc Chromate: Zinc chromate is produced by reacting an aqueous slurry of zinc oxide or hydroxide with dissolved chromate ions followed by neutralization, or by precipitation of dissolved zinc salts with dissolved chromate salts.
Zinc tetraoxychromate is produced from zinc oxide and chromic acid in an aqueous medium. Basic zinc potassium chromate is obtained by reacting an aqueous slurry of zinc oxide with potassium dichromate and sulfuric acid.
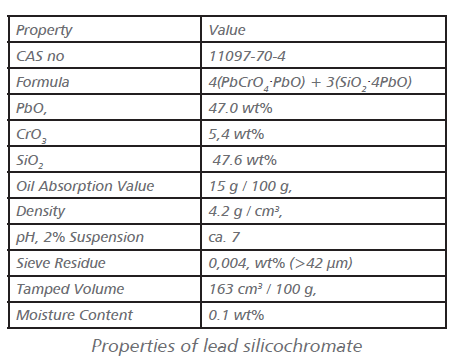
The pigments are washed, filtered, dried. and ground. Strontium Chromate: Strontium chromate is precipitated from Solutions of sodium dichromate and strontium chloride, followed by filtration, washing, drying, and grinding. Lead Silicochromate: Lead silicochromate is an orange powder.
This pigment is a core pigment, in which the active pigment substance (PbCrO4) is precipitated onto an inert core (SiO2).
Anticorrosive Molybdate Pigments: Molybdenumbased anticorrosive pigments offer a nontoxic alternative to the zinc chromate pigments. They all have a neutral color (white) but the pure compounds are very expensive.
To produce economically competitive pigments molybdate and phosphate pigments are combined, or molybdate compounds are applied to inorganic fillers (e.g., zinc oxide, alkaline-earth carbonates, or talc).
Phosphate-containing molybdate pigments are especially suitable for water-thinnable or latexbased binders, because they improve adhesion to iron substrates. The other molybdate pigments are mainly used in solvent borne binder systems.
Unlike chromate ions in chromate pigments, the MoO2-4 ions in molybdate pigments are not chemically reduced in most coatings. Hence, they are ineffective for cathodic protection.
Their protective action is assumed to be due to activity in the anodic region, similar to that of phosphate ions. As with the protective phosphate fılms, molybdate fılms are very resistant to chloride and sulfate. The duration of maximum activity depends on the metal ions used in the pigment and is probably due to differences in solubility.
Anticorrosive Lead and Zinc Cyanamides: Lead cyanamide is a lemon yellow powder. Zinc cyanamide is a white to beige powder. The cyanamides are active anticorrosive pigments which have a passivating action under alkaline conditions.

The main fields of application are in mirror coatings, electrodeposition coatings, and primers cyanamide pigments are produced from industrial-grade calcium cyanamide which is first dissolved. Sulfide and phosphide impurities are precipitated as iron or lead salts or oxidized and fıltered off together with graphite impurities.
The pure calcium cyanamide is reacted in an aqueous medium with soluble lead or zinc salts or with a slurry of lead oxide or zinc oxide. The pigments are filtered, washed, dried, and ground. Zinc cyanamide and pure lead cyanamide are not explosive.
An explosion reported during the production of lead cyanamide was caused by contamination with small amounts of acid or nitrates. Zinc cyanamide is nontoxic, but the toxicological classification of lead cyanamide has to take its lead content into account.
Ion-Exchange Pigments: Ion-exchange pigments were developed as nontoxic alternatives to the chromate pigments. They consist of a silicate carrier (zeolite or amorphous silica gel) to which calcium ions are bound. Commercial ion-exchange pigments have the following properties.
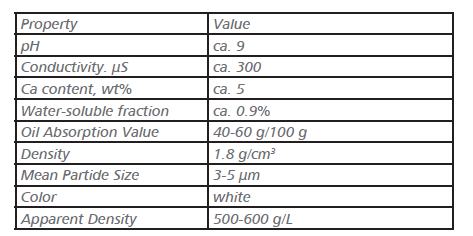
Ion-exchange pigments should be classified as active anticorrosive pigments. They act by exchanging their calcium ions for hydrogen ions in the paint film. In this way acidic chemical sub- stances are neutralized. The calcium ions then bind to the metal oxide surface.
The pigments have good anticorrosive properties owing to their high pH value, but the danger of pinholing (depending on the binder) should be pointed out.
Anticorrosive Metal Oxide Pigments
Red Lead: Red lead, crystallizes in the tetragonal system and is a red powder. It decomposes at ca. 500°C at atmospheric pressure. Red lead should be regarded as the lead salt of orthoplumbic acid, H4PbO4 i.e., it is lead(II) orthoplumbate, Pb2PbO4, in which PbO6 octahedra are linked by Pb(II) ions.
Red lead is produced industrially by oxidizing lead monoxide (PbO) at ca. 460-480°C with agitation in a stream of air for 15-24 h. Most red lead is used in the glass, ceramic, and accumulator industries where an apparent density of <2 g/mL is adequate.
For the paint industry, however, highly dispersed red lead is normally necessary, with a sieve residue of <0.1% on a 0.063 mm sieve and an apparent density of 1.3-2.0 g/mL
The electrochemical action of red lead results from the fact that lead has valences of 2 and 4 in lead orthoplumbate: Pb (IV) compounds are reduced to Pb (II) in the cathodic region. The chemical anticorrosive effect is a result of lead soaps that are formed when fatty acids in the binder react with the red lead.
The lead soaps permeate the paint film as lamellae, and give good mechanical strength, water resistance, and adhesion to the Steel surface. Furthermore, the corrosion-promoting chloride and sulfate ions are precipitated by lead (II) ions.
Red lead is still used for heavy-duty anticorrosion applications, especially for surfaces bearing residual traces of rust. In waterborne paints, red lead has no advantages over zinc phosphate.
Calcium Plumbate: Calcium plumbate, Ca2PbO4, density 5.7 g/cm3, is a beige powder formed from lead monoxide and calcium oxide at ca. 750°C in a stream of air. The anticorrosive properties of calcium plumbate are inferior to those of red lead.
Calcium hydroxide is formed as a hydrolysis product when water penetrates through a primer that contains calcium plumbate. The pH at the metal surface then increases to ca. 11-12 which inhibits corrosion.
The most important use is in primers for zinc- coated substrates. The pH change occurring on hydrolysis of the calcium plumbate etches the zinc surface which improves adhesion of primers, especially on hot-dip galvanized steel.
Anticorrosive Zinc and Calcium Ferrites: Many paint formulations contain iron oxide as an extender. It is a physically protective anticorrosive pigment (only to a small extent). In order to obtain a chemically protective anticorrosive pigment with active constituents the iron oxide is heated with oxides or carbonates of alkaline earths (CaO, CaCO3) or zinc (ZnO) to form pigments of the ferrite type.
In the coating these pigments are hydrolyzed with water to form alkaline-earth hydroxides or zinc hydroxide which prevent corrosion by increasing the pH. Alkaline-earth soaps are also formed in certain binder media.
However, the pigment volume concentration must be high to ensure good results. Only one zinc ferrite pigment has attained economic signifıcance. Its properties are as follows:
Zinc Oxide: Zinc oxide, is a white powder that is usually used in combination with active anticorrosive pigments. The inhibiting action of zinc oxide is based on its ability to react with corrosive substances and to maintain an alkaline pH in the coating. It also reacts with acidic components of the binder to form soaps and absorbs UV light.
The lead content of commercial zinc oxide depends on the manufacturer and is in the range 0.002- 1.5%. For a zinc oxide coating to be considered leadfree, the lead content must be less than 1.5%.
Anticorrosive Powdered Metal Pigments
Zinc Dust: Zinc dust, is a free-flowing blue-gray powder composed of spheroidal particles. It is produced by melting zinc in a crucible, vaporizing it at ca. 900-950°C, and condensing and sifting the product. Alternatively, molten zinc is atomized with a nozzle to produce dust, which is then sifted.
The action of zinc dust in primers with organic binders is based on sealing effects and electro- chemical processes. The zinc reacts with water and atmospheric oxygen that diffuse into the binder, reziforming zinc hydroxide which is then neutralized by sulfuric acid (from SO2 in the air) and hydrochloric acid (from Cl-containing substances in the air, e.g., NH4C1).
This causes an increase in volume and decreases permeability. The corrosion products of zinc also have an anticorrosive action. Cathodic protection takes place when the zinc and iron come into contact; the zinc content in the primer must be at least 94-96%.
Zinc dust coatings are used in large quantities for structural Steel, including under water steel construction and shipbuilding. Zinc dust is also used in inorganic binder systems (alkali silicates or alkyl silicates) in the form of two-component systems.
Lead Powder: Lead powder, is a dark gray powder containing 99% metallic lead and ca. 0.5% lead (II) oxide. It is produced by spraying molten lead and then sifting. Owing to its high surface area (particle size 1-15 μm), it is liable to oxidize, and is therefore supplied in airtight packages or as a paste.
Lead powder can be combined with many binders. It does not affect the stability or viscosity of the paint. Binders that absorb only small amounts of water are particularly suitable (e.g., epoxy resins, chlorinated rubber). When formulating paints based on lead powder, care must be taken not to dilute it with other pigments and extenders by more than 5 vol%.
Lead powder coatings are mainly used for protecting against aggressive chemicals. They have a high UV reflection and extremely good elasticity. Lead powder pigments and pastes are also used in radiological protection.
Anticorrosive Flake Pigments: They increase the barrier resistance of a coating towards water and aggressive gases by increasing the length of the diffusion path in the coating.
The interaction between pigment and binder should be as water-resistant as possible to prevent the diffusion of water from the surface of the flakes through the coating.
In principle, all lamellar minerals may be used as barrier pigments, e.g., micaceous iron ore, layer silicates (mica), linear polymeric silicates (wollastonite), and talc. However, untreated mica and talc are not very suitable because they are highly permeable to water.
The surface can be modified with, for example, silanes or titanates, to reduce water permeability and improve adhesion. If the chemistry of the treated pigment surface is adapted to the functional groups of the binder, the pigment can be used in aqueous anticorrosive dispersions.
Flake aluminum pigments with varying platelet thicknesses and shapes are used for corrosion protection. They are coated with a water-repellent, fatty film and are therefore particularly suitable for conventional solvent borne coating systems. They have outstandingly good weather resistance.
Flake zinc pigments have a barrier effect and also act by a cathodic anticorrosive mechanism. Compared with zinc dust coatings, flake zinc pigments are formulated with lower pigment volume concentrations.
Anticorrosive Organic Pigments: Unlike soluble inhibitors, organic anticorrosive pigments are sparingly soluble organic com- pounds or metallic salts of organic acids and are used in binders instead of, or in addition to, inorganic anticorrosive pigments. inhibitors are not viable substitutes for active anticorrosive pigments in coatings.
Organic anticorrosive pigments were developed to replace toxic, chromate-based anticorrosive pigments.
The Zinc Salt Of 5-Nitroisophthalic Acid: The zinc salt of 5-nitroisophthalic acid is produced as an easily dispersible pigment by a wet chemical process from 5-nitroisophthalic acid and zinc oxide,
A less expensive replacement for zinc chromate is obtained by combining the zinc salt of 5-nitroisophthalic acid with zinc phosphate pigments, A concentration of 0.5-2.0% based on the liquid coating is recommended.
The electrochemical anticorrosive action of the zinc salt of 5-nitroisophthalic acid is comparable with that of zinc potassium chromate. The compound is also nontoxic.
Other Organic Anticorrosive Pigments: The following organic anticorrosive pigments are either commercial products or have been reported in the literature: • (2-Benzothiazolylthio) succinic acid, • Zinc mercaptobenzothiazole, • Basic zinc salt of A-benzosulfonylanthranilic acid.
They are used in the binder at concentrations of 0.5- 2.0%, if possible, in combination with zinc phosphate or other suitable anticorrosive pigments.
M. Namık Kayaalp – Chemical Engineer – Ecelak Boya Kimya San. Tic. Ltd. Şti.
References
1) ICL Advanced Additives, a division of ICL Specialty Products Inc. 2018
2) Industrial Inorganic Pigments, Dr. Gunter Buxbaum, 47812 Krefeld Germany, Dr. Gerhard Pfaff, 64271 Darmstadt Germany, Revised Edition 2005
3) Smith, A. Inorganic Primer Pigments, Federation Series on Coating Technology, PA 1988,
4) Paint Formulation, J.Boxall, G.Godwin Ltd.-London 1980
5) Organic Coating Technology-II, H.F.Payne, J.Wiley and Sons Inc.-Newyork 1960
6) Oil, Colours Chemists’Association-London 1966
7) Ullmann’s Encyclopedia of industrial Chemistry, H. Wienand, Vol. A20, Sec. 3.3, VCH Inc.
8) Paint and Coating Testing Manual 15. Edition of the Gardner-Sward Handbook, Joseph V. Koleske,





