The conditioning polymers are effective skin and hair modifiers because they are designed to deposit, adhere, adsorb, or absorb to proteinaceous elements of the hair and skin. In the personal care industry, the term “substantivity” describes the binding of a material to these surfaces. To be effective conditioners, however, it is not sufficient for the materials simply to adhere to the skin and hair. They must contribute some attribute that the consumer perceives as beneficial to these surfaces. lndeed, conditioning effects are among the most important characteristics the consumer seeks when purchasing a personal care product.
This article covers one types of synthetic conditioning polymers, cationic polymers.
Synthetic Conditioning Polymers
Table 1 shows the structure and ıncı designation for many of the cosmetically useful synthetic, cationic, conditioning polymers. The structures are separated by the nature of the cationic moiety, which distinguishes each class of cationic polymer. It is clear that only a few monomers that carry cationic charge are currently employed in the industry. Most of the polymers are the product of addition polymerization reactions employing unique acrylate or vinyl monomers.
The structures ofthese polymers is deterrnined by the coreactivity of the monomers. A variety of terms, some of them confusing, are used in the patent and open literature to describe the “amount” of cationic charge on the cationic polymer. Table 2 lists three of the most common terms. The distinction of cationic nitrogen from other forms of noncationic nitrogen in these polymers is important. For instance, both Kjeldahl and combustion analysis, which report the combination of cationic nitrogen and other nitrogen, can be applied to Polyquatemium-7. Only the cationic nitrogen is, however, important in these charge calculations.
It is also important to realize that some nitrogen-containing monomers are cationic only if the formulation pH is sufficiently low to protonate the nitrogen group. As an example, Polyquatemium- 16 is comprised of a quatemary vinyl imidazole monomer attached to the polymer backbone through a tertiary vinyl amine. Although the quatemary nitrogen is almost always positively charged, the tertiary nitrogen may or may not be, depending on the pH of the formulation. When calculating charge on this polymer, the formulator must consider that the tertiary nitrogen might contribute to the polymer’s cationicity.
Cationic substitution (CS) is a dimensionless descriptor that tells the formulator something about the moles of cationic substituent per mole of polymer. Charge density is calculated from CS and defined as rnilliequivalents per gram (mEq/g). It describes the amount of cationic charge per gram of polymer. A polymer with a charge density in the range of 3-6 is highly charged. Poly(ethyleneirnine) is reported to have a charge density of nearly 20 at pH 4.5. polymers with a charge density below 1.0 are lightly cationic.
There has been very little quantitative study of the deposition of synthetic polymers onto proteinaceous substrates . The most successful methods for measuring this have been radiolabeling and electrokinetic measurements.
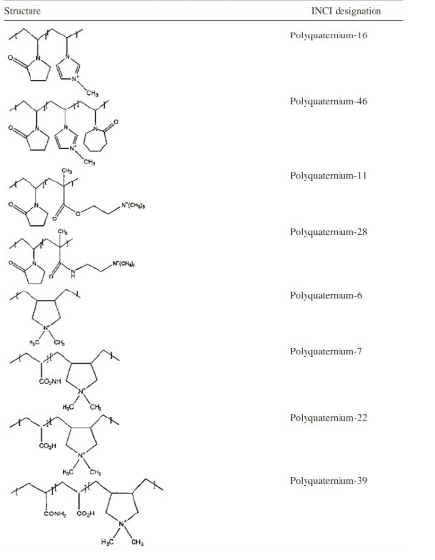
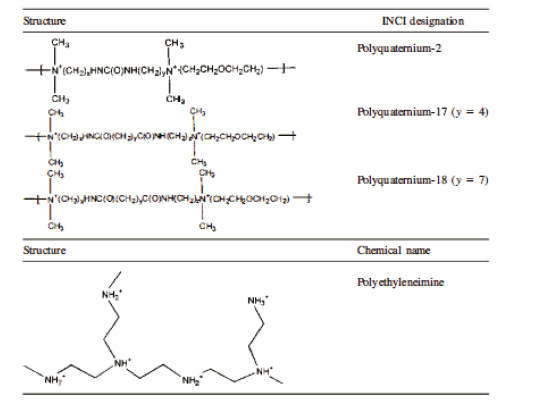
Table 1. Structure and INCI Designation or Chernical Name for Several Synthetic, Cationic, Conditioning Polymers
Radiolabeling is particularly effective, but the use of radioactive polymers is discouraged in most research institutions. The signal- swamping effect from the amitle and primary nitrogens that comprise the polypeptide proteins of hair and skin hinders several spectroscopic methods.
Any technique employed to quantitate deposition must be able to exclude the results from these proteinaceous nitrogens. Despite these quantitative challenges, however, there is little question that cationic polymers have a strong affection for skin and hair. More subjective analyses show that deposition of cationic polymers onto hair and skin elicits improvements in the structure and appearance ofthese surfaces.
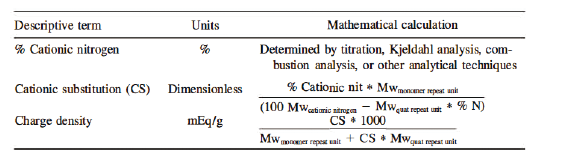
Table 2. Methods for Describing Level of Cationic Charge on a Polymer
Such techniques, however, can not distinguish, for example, the conditioning effect of a cationic polymer from that of a low-molecular- weight cationic conditioner. Careful control experiments are required to isolate effects from the polymer alone. The cationic charge on a polymer affects it is behavior considerably when surfactants are included in formulations. Cationic polymers (a member of the broader class of polymers termed polyelectrolytes) usually interact strongly with anionic surfactants, weakly with cationic surfactants, and unpredictably with nonionic and amphoteric surfactants Anionic surfactant binds to cationic polymers at concentrations well below he crtical micelle concentration (eme).
The low surfactant concentration where polymer and surfactant begin to interact is known as the critical aggregation concentration (cag). As the anionic surfactant concentration increases, more of the polymer’ s cationic sites complex with surfactant. The developing charge forces polymer chains to elongate, which in turn improves chain entanglement. Also the developing surfactant micelles promote interpolymer cross-linking. This, together with the increased chain entanglement, causes an increase in viscosity.
This effect is dependent on the concentration of the polymer, which must be in the concentrated regime. With the addition of even more surfactant, neutralization of the available cationic charge occurs and the polymer and surfactant will form a complex phase known as a coacervate. At the extreme, the polymer/ surfactant complex may become water-insoluble and precipitate. Toese precipitates are termed ‘’polyelectrolyte complexes” (PEC). The development of a surfactant/polymer coacervate or PEC depends on other factors including, for example, the chain length of surfactant hydrophobes and the presence of electrolytes such as salt.
It is felt that the presence of the polymer/surfactant coacervate is important for deposition of the polymer onto the anionic surfaces of hair and skin. It has been demonstrated that when a cationic polymer is deposited onto hair as a coacervate, subsequent rinsing removes the anionic surfactant more quickly than the bound polymer. In these electokinetic measurements, the overall charge of the hair gradually becomes more cationic. It should be kept in mind, also, that coacervate can form when a concentrated anionic surfactant solution containing a solubilized cationic polymer is diluted by the addition of water. This is the typical mode of application of conditioning shampoos to hair and skin during washing. The deposition occurs during the rinsing cycle and is appropriately termed “dilution deposition.”
Polyelectrolyte complexes have fund less practical applications in personal care, principally because they are so water-insoluble. However, they are extremely useful as drug delivery vehicles. Recently, it was demonstrated, for instance, that a PEC formed between polymers like Polyquaternium-6 and retinoic acid (Vitamin D), a powerful skin exfoliating agent, helps to stabilize the labile vitamin, prolonging its chemical life and potentially increasing it is potency.
The developınent of charge-neutral coacervate is linear in polymer and surfactant concentration. At surfactant concentrations beyond those required to neutralize the cationic polymer, the coacervate disappears and the surfactant usually, but not always, solubilizes the polymer. Polymers with higher charge density are more difficult to solubilize, and polyelectrolyte complexes will, typically, not redissolve. The behavior of cationic polymers in the presence of highly concentrated surfactant solutions, where the micelles are no longer spherical but instead can exist as rod-like and branching structures, has not been adequately addressed. In such a strongly anionic environınent, the rheological influence of the cationic polymers is likely minimal.
References
1. Kuhn T. Structure of Scientific Revolutions, 2nd ed. Chicago: University Press, 1970.
2. Doi Y. Microbial Polyesters. New York: VCH Publishers, 1990.
3. Burdick DL, Leffler WL. Petrochernicals in Nontechnical Language, 2nd ed. Tulsa, OK: Penn W ell Publishing, 1990.
4. Volk H, Friedrich RE. Polyacrylarnide. in: Davidson RL, ed. Handbook of Water-Soluble Gums and Resins. New York:
McGraw-Hill, 1980: Chapter 16, 1-19.

Ömer Arif Kural
Foreign Trade Manager
Cosmer Kimya A.Ş.





